Metal-free transesterification catalyzed by tetramethylammonium methyl carbonate†
Abstract
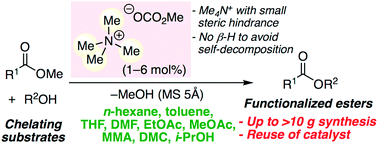
Environmentally benign metal-free tetramethylammonium methyl carbonate is effective as a catalyst for the chemoselective, scalable, and reusable transesterification of various esters and alcohols in common organic solvents. In situ-generated highly active species, tetramethylammonium alkoxides, can greatly avoid self-decomposition at ≤110 °C, and are reusable. In particular, chelating substrates, such as amino alcohols, diols, triols, sugar derivatives, alkaloids, α-amino acid esters, etc., which deactivate conventional metal salt catalysts, can be used. A 100 gram scale biodiesel production was also demonstrated.


 Please wait while we load your content...
Please wait while we load your content...