Efficient synthesis of 1,2-benzisothiazoles from o-haloarylamidines and elemental sulfur via N–S/C–S bond formation under transition-metal-free conditions†
Abstract
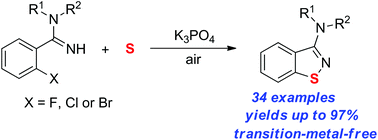
An efficient method for the synthesis of 1,2-benzisothiazoles from amidines and elemental sulfur via N–S/C–S bond formation under transition-metal-free conditions is described. This reaction is performed smoothly under simple conditions to give the corresponding products in good to high yields with good functional group tolerance.


 Please wait while we load your content...
Please wait while we load your content...