Lewis pairs for ring-opening alternating copolymerization of cyclic anhydrides and epoxides†
Abstract
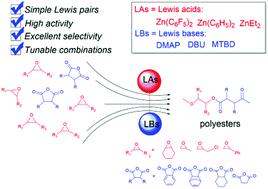
A simple and highly active catalytic process for ring-opening alternating copolymerization (ROAC) of cyclic anhydrides and epoxides still remains a key challenge. Herein, we have described an effective group of versatile and low-toxic zinc dicarbyl/amine Lewis pairs for the ROAC. The facile route showed a high catalytic activity (TOF ≤ 210 h−1 at 110 °C) and perfectly alternating selectivity (>99%). An unexpected highly regioselective ring-opening of asymmetric epoxides (PO, ECH and SO) was also achieved by the combination of zinc alkyls (or aryls) and amines. Of note, deprotonation side reaction of α-H of anhydrides with organic bases was uncovered, and subsequently was inhibited by using nonpolar solvents and Lewis acid/base pairs. Thus, an array of polyesters was synthesized by the coupling of various anhydrides (PA, CHA, SA and NA) and epoxides (CHO, PO, ECH and SO) using the same Lewis pairs. Furthermore, variable temperature 1H NMR spectral and MALDI TOF MS analyses were performed to understand the possible mechanism and microstructure. The experimental results indicated that zwitterionic alkoxide and carboxylate intermediates alternately formed to enhance the ester repeat units in chain initiation and propagation. This work provides a simple and green catalytic strategy to prepare diversified polyesters from the ROAC process of cyclic anhydrides and epoxides with considerable catalytic activity and alternating selectivity.
- This article is part of the themed collection: 2018 Green Chemistry Hot Articles


 Please wait while we load your content...
Please wait while we load your content...