Purification of crude In(OH)3 using the functionalized ionic liquid betainium bis(trifluoromethylsulfonyl)imide†
Abstract
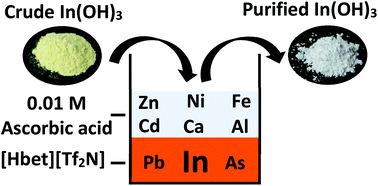
The recovery of indium from a crude indium(III) hydroxide using the ionic liquid betainium bis(trifluoromethylsulfonyl)imide, [Hbet][Tf2N], was investigated. Leaching and solvent extraction were combined in one step using the thermomorphic properties of the [Hbet][Tf2N]–H2O system. During leaching (80 °C) a homogeneous phase was formed. Upon lowering the temperature below the lower critical solution temperature (UCST), the dissolved metals distributed themselves between the two phases. The optimal leaching/extraction conditions were determined to be a leaching time of 3 hours at 80 °C in a 1 : 1 wt/wt [Hbet][Tf2N]–H2O mixture. Large separation factors (>100) between In(III) and Al(III), Ca(II), Cd(II), Ni(II) and Zn(II) were obtained implying an easy separation. Fe(III), As(V) and Pb(II) are co-extracted. The separation factor between indium and iron was improved to >1000 by addition of ascorbic acid to reduce Fe(III) to Fe(II). The stripping was done very efficiently by HCl solution. The ionic liquid was regenerated during the stripping step. By combining a prehydrolysis and hydrolysis step, indium(III) hydroxide with a purity >99% was obtained.


 Please wait while we load your content...
Please wait while we load your content...