A new route to α,ω-diamines from hydrogenation of dicarboxylic acids and their derivatives in the presence of amines†
Abstract
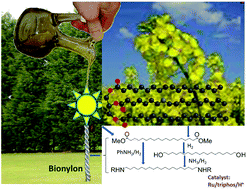
A new and selective route for the synthesis of polymer precursors, primary diamines or N-substituted diamines, from dicarboxylic acids, diesters, diamides and diols using a Ru/triphos catalyst is reported. Excellent conversions and yields are obtained under optimised reaction conditions. The reactions worked very well using 1,4-dioxane as solvent, but the greener solvent, 2-methyl tetrahydrofuran, also gave very similar results. This method provides a potential route to converting waste biomass to value added materials. The reaction is proposed to go through both amide and aldehyde pathways.


 Please wait while we load your content...
Please wait while we load your content...