A base-free, ultrasound accelerated one-pot synthesis of 2-sulfonylquinolines in water†
Abstract
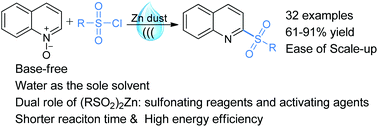
Without employing any base and organic solvent, an economical, practical and eco-friendly protocol has been developed for the one-pot synthesis of various functionalized 2-sulfonylquinolines from easily accessible starting materials in water under open-air conditions. Compared with conventional heating conditions, the use of ultrasound techniques not only improves the reaction efficiency and enhances the reaction rate but also minimizes the side reactions. This process has a broad substrate scope, mild reaction conditions, good yields, excellent chemo- and regioselectivities, operational simplicity, ease of scale-up and high energy efficiency. In addition, the process is remarkably greener than the previous routes with an atom economy of 70.7%, an E-factor of 1.17 and an eco-scale score of 71.


 Please wait while we load your content...
Please wait while we load your content...