Isomerization of glucose to fructose catalyzed by lithium bromide in water†
Abstract
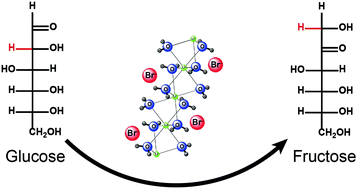
This study demonstrated that glucose could be isomerized to fructose in the concentrated aqueous solution of lithium bromide (LiBr) under mild conditions. The isomerization mechanisms were studied via isotopic labeling experiments. It was found that both the cation (Li+) and the anion (Br−) in the system catalyzed the isomerization of glucose to fructose. Br− catalyzed the isomerization through the proton transfer mechanism via an enediol intermediate, while Li+ did it through an intramolecular hydride shift mechanism from C2 to C1. The estimation using quantitative 13C-NMR analysis indicated that Br− catalyzed approximately 85% of the isomerization, while Li+ was responsible for the remaining 15%. The results indicated that 31% of the fructose was produced from glucose in LiBr trihydrate at 120 °C for 15 min. The outcomes of this study provided not only a better understanding of and insights into the sugar transformations in the LiBr solution but also an alternative approach to produce fructose from glucose.


 Please wait while we load your content...
Please wait while we load your content...