Catalytic hydrogenolysis of kraft lignin to monomers at high yield in alkaline water†
Abstract
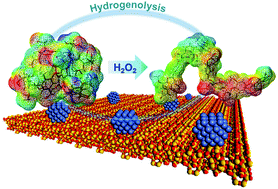
Inspired by the results of calculation on the basis of density functional theory and a semi-empirical method, we found an easy, robust, and efficient approach to solve the problem of folded lignin macromolecules, which is a key factor for impeding their breakdown into monomers by hydrogenolysis. Oxidation and hydrogenolysis, which appear to be independent and contradictory of each other in many past studies, were combined and successively performed in this study. Hydrogen peroxide was used to damage the strong intramolecular hydrogen bonds of kraft lignin efficiently, transforming the folded three-dimensional geometries of the lignin macromolecules into stretched ones in an alkaline aqueous medium. Following the pretreatment of stretching lignin molecules, catalytic hydrogenolysis was performed in the presence of a Ni catalyst supported by the ZSM-5 zeolite, reported by the authors. Because of more chemisorption sites of the stretched lignin macromolecules onto the catalyst surface and the remission of lignin re-polymerization/self-condensation, conversion of the kraft lignin into oil reached 83 wt% lignin, 91 wt% which was accounted for by nine types of monomers. This study has thus demonstrated high yield monomer production from lignin dissolved in aqueous media.


 Please wait while we load your content...
Please wait while we load your content...