Highly effective Ir-based catalysts for benzoic acid hydrogenation: experiment- and theory-guided catalyst rational design†
Abstract
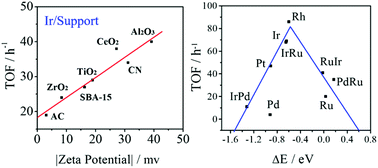
On the way to exploring superior hydrogenation catalysts, Ir-based catalysts with a record catalytic activity (up to 40 h−1) for the hydrogenation of benzoic acid to cyclohexanecarboxylic acid under mild reaction conditions (85 °C, 0.1 MPa H2, in water) have been successfully developed. By excluding various factors, the experimental results showed that the main factor governing the activity discrepancy between the Ir-based catalysts is actually the dispersion stability of the supports (such as N-doped carbon, active carbon, SBA-15 and various metal oxides) in the reaction solution, rather than the interaction between the Ir active component and the supports. Combined with theoretical investigation from first principles, an activity volcano curve considering the competing adsorption between the reactants (H2) and solvent (H2O) for aqueous aromatic ring hydrogenation was presented for the first time. The high activity of Ir can be deduced by the proper discrepancy of dissociation energies or adsorption energies between H2 and H2O on the catalysts. This activity volcano curve provides guidance for further rational design of promising catalysts for benzoic acid or even aromatic ring hydrogenation under true reaction conditions for practical applications.


 Please wait while we load your content...
Please wait while we load your content...