Water-controlled selective preparation of α-mono or α,α′-dihalo ketones via catalytic cascade reaction of unactivated alkynes with 1,3-dihalo-5,5-dimethylhydantoin†
Abstract
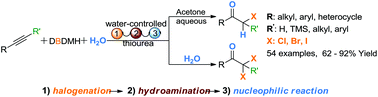
The control of a reaction that can produce multiple products from the same starting material is a highly attractive and challenging concept in organic synthesis. An efficient protocol for the selective synthesis of α-mono or α,α′-dihalo ketones via a water-controlled three-component thiourea-catalyzed cascade reaction of unactivated alkynes, 1,3-dihalo-5,5-dimethylhydantoin and water has been developed. α-Monohaloketones were obtained in aqueous acetone at 45 °C; conversely, α,α′-dihalo ketones were formed with pure water as the sole solvent at room temperature.


 Please wait while we load your content...
Please wait while we load your content...