Nucleophilic ring opening of aziridines with amines under catalyst- and solvent-free conditions†
Abstract
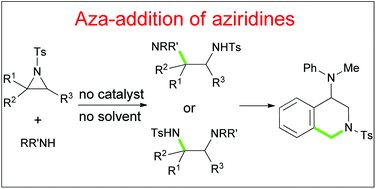
The aza-addition of aziridines to obtain vicinal-diamines was achieved under catalyst- and solvent-free conditions for the first time. Various aryl, alkyl and meso-bicyclic aziridines with a Ts-protecting group were tolerated, and aromatic amine-nucleophiles containing electron withdrawing and donating groups in the phenyl ring were applied. The plausible reaction mechanism involving hydrogen bonding and proton transfer was proposed. The synthetic utility of one addition adduct to the 4-amino-1,2,3,4-tetrahydroisoquinoline derivative through the Pictet–Spengler reaction was realized.


 Please wait while we load your content...
Please wait while we load your content...