A facile and efficient route to hydrophilic ionic liquids through metathesis reaction performed in saturated aqueous solution†
Abstract
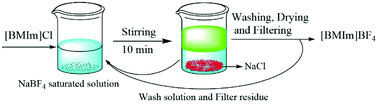
The preparation of ionic liquids most often involves the use of organic solvents or hazardous chemicals, especially for hydrophilic ionic liquids prepared from metathesis reaction. In this study, the salting-out effect was proposed to promote the metathesis reaction to afford water-miscible ionic liquids. The reaction proceeds in aqueous solution saturated with an inorganic precursor salt. After reaction for only 10 min, the hydrophilic product can be easily obtained by spontaneous liquid–liquid phase-separation caused by the salting-out effect, followed by concentration and filtration. In this process, no organic solvents or hazardous chemicals are required, and the saturated solution and inorganic by-product can be easily reused and recycled respectively.


 Please wait while we load your content...
Please wait while we load your content...