Highly efficient formic acid-mediated oxidation of renewable furfural to maleic acid with H2O2†
Abstract
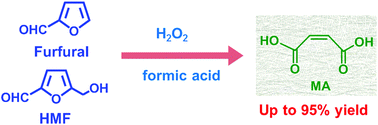
Maleic acid and its anhydride are important intermediates in the chemical industry produced on a multimillion tonne-scale annually. The synthesis of maleic acid/anhydride from renewable biomass resources such as furfural and 5-hydroxymethylfurfural is highly desirable for the sustainability of human society. Most of the previously reported processes for maleic acid/anhydride synthesis from biomass suffer from low efficiency, complicated conditions and poor catalyst recyclability. Herein, we demonstrate a highly efficient and simple system for the synthesis of maleic acid from furfural. An excellent yield (95%) of maleic acid was achieved under mild conditions in this very simple system which requires only H2O2 as an oxidant in formic acid solvent. Under similar conditions, an 89% yield of maleic acid was achieved from biomass-derived 5-hydroxymethylfurfural. This study presents a novel synthetic method and a promising process for maleic acid production from renewable biomass resources.


 Please wait while we load your content...
Please wait while we load your content...