One-pot, two-step cascade synthesis of naturally rare l-erythro (3S,4S) ketoses by coupling a thermostable transaminase and transketolase†
Abstract
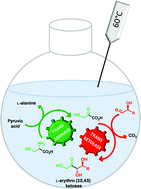
An efficient simultaneous cascade of two enzymatic steps catalyzed by a thermostable transaminase and transketolase was performed at elevated temperatures allowing the synthesis of naturally rare L-erythro (3S,4S) ketoses. L-ribulose, 5-deoxy-L-ribulose, D-tagatose and L-psicose, which are highly valuable chiral building blocks and display prominent biological properties, were obtained on a preparative scale with excellent stereoselectivities and good yields. A thermostable transketolase from Geobacillus stearothermophilus catalyzed at high temperatures the stereospecific synthesis of L-erythro (3S,4S)-configured ketoses from (2S)-hydroxylated aldehydes and β-hydroxypyruvate in which the latter is generated in an unprecedented manner in situ from natural L-serine and pyruvate using a novel thermostable L-α-transaminase from the thermophilic bacterium Thermosinus carboxydivorans. Overall, this cascade synthesis prevents the thermal decomposition of the labile β-hydroxypyruvate and offers an efficient and environmentally friendly procedure.
- This article is part of the themed collections: 2017 Green Chemistry Hot Articles and Enzyme catalysis in organic synthesis

 Please wait while we load your content...
Please wait while we load your content...