Metal-free annulation/aerobic oxidative dehydrogenation of cyclohexanones with o-acylanilines: efficient syntheses of acridines†
Abstract
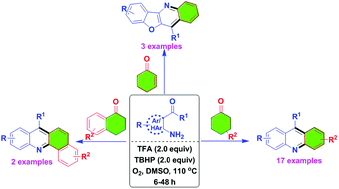
We have identified metal-free reaction conditions for the annulation/aerobic oxidative dehydrogenation of cyclohexanones with o-acylanilines to the corresponding acridine derivatives. The combination of trifluoroacetic acid (TFA), tert-butyl hydroperoxide (TBHP), dimethylsulfoxide (DMSO) and oxygen (O2) converted the cyclohexanone derivatives to an aromatic aryl product in moderate to good yields. The experimental results suggest that this process involves an aza-allyl oxidation intermediate.

 Please wait while we load your content...
Please wait while we load your content...