Cleavage of the lignin β-O-4 ether bond via a dehydroxylation–hydrogenation strategy over a NiMo sulfide catalyst†
Abstract
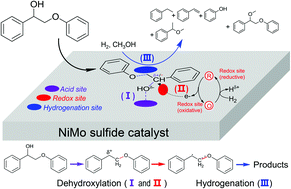
The efficient cleavage of lignin β-O-4 ether bonds to produce aromatics is a challenging and attractive topic. Recently a growing number of studies have revealed that the initial oxidation of CαHOH to Cα![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O can decrease the β-O-4 bond dissociation energy (BDE) from 274.0 kJ mol−1 to 227.8 kJ mol−1, and thus the β-O-4 bond is more readily cleaved in the subsequent transfer hydrogenation, or acidolysis. Here we show that the first reaction step, except in the above-mentioned pre-oxidation methods, can be a Cα–OH bond dehydroxylation to form a radical intermediate on the acid-redox site of a NiMo sulfide catalyst. The formation of a Cα radical greatly decreases the Cβ–OPh BDE from 274.0 kJ mol−1 to 66.9 kJ mol−1 thereby facilitating its cleavage to styrene, phenols and ethers with H2 and an alcohol solvent. This is supported by control experiments using several reaction intermediates as reactants, analysis of product generation and by radical trap with TEMPO (2,2,6,6-tetramethyl-1-piperidinyloxy) as well as by density functional theory (DFT) calculations. The dehydroxylation–hydrogenation reaction is conducted under non-oxidative conditions, which are beneficial for stabilizing phenol products.
O can decrease the β-O-4 bond dissociation energy (BDE) from 274.0 kJ mol−1 to 227.8 kJ mol−1, and thus the β-O-4 bond is more readily cleaved in the subsequent transfer hydrogenation, or acidolysis. Here we show that the first reaction step, except in the above-mentioned pre-oxidation methods, can be a Cα–OH bond dehydroxylation to form a radical intermediate on the acid-redox site of a NiMo sulfide catalyst. The formation of a Cα radical greatly decreases the Cβ–OPh BDE from 274.0 kJ mol−1 to 66.9 kJ mol−1 thereby facilitating its cleavage to styrene, phenols and ethers with H2 and an alcohol solvent. This is supported by control experiments using several reaction intermediates as reactants, analysis of product generation and by radical trap with TEMPO (2,2,6,6-tetramethyl-1-piperidinyloxy) as well as by density functional theory (DFT) calculations. The dehydroxylation–hydrogenation reaction is conducted under non-oxidative conditions, which are beneficial for stabilizing phenol products.


 Please wait while we load your content...
Please wait while we load your content...