Alkyl melibioside and alkyl cellobioside surfactants: effect of sugar headgroup and alkyl chain length on performance†
Abstract
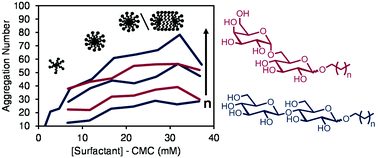
The potential of glycolipid surfactants, composed of a sugar headgroup and lipid tail, as highly biodegradable and less toxic alternatives to commonly used surfactants motivates the systematic study of structure–function relationships of various glycolipid surfactants. Advances in the efficient synthesis of high purity amphipathic glycolipid surfactants enable the analysis of a diverse set of glycolipids. The solution phase properties of two suites of glycolipid surfactants, n-alkyl-O-melibiosides and n-alkyl-O-cellobiosides, with varying straight-chain alkyl tails of 8, 10 and 12 carbons are investigated in this work. This study substantiates their efficient surfactant performance and reveals their aggregate structure and microenvironment as it relates to molecular structure. Surface tensiometry demonstrates critical micelle concentrations of 0.2–40 mM with minimum surface tension values of 36–40 mN m−1. Time resolved fluorescence quenching (TRFQ) spectroscopy is used to calculate micelle aggregation number (12–70 molecules per micelle) and assess relative micropolarity and microfluidity within the aggregates. The average aggregation numbers determined by TRFQ and apparent hydrodynamic radii determined by dynamic light scattering vary with alkyl chain length and headgroup size, but these properties are relatively insensitive to changes in concentration. The aggregates form nearly spherical, tightly-packed micelles with a small population of vesicles and polydispersity present at low concentrations or with short alkyl chain length.
- This article is part of the themed collection: Molecular Design for Reduced Toxicity

 Please wait while we load your content...
Please wait while we load your content...