Sulfination of alcohols with sodium sulfinates promoted by BF3·OEt2: an unexpected access†
Abstract
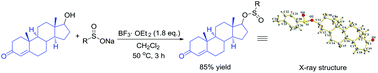
A BF3·OEt2-promoted direct substitution of various alcohols with sodium sulfinates affording sulfinates under mild conditions has been developed. Further reaction of the hydroxysteroids achieves the highly complex sulfinates in good yields, which are two potential pharmacophores routinely encountered in drug discovery.

 Please wait while we load your content...
Please wait while we load your content...