Light driven styrene epoxidation and hydrogen generation using H2O as an oxygen source in a photoelectrosynthesis cell†
Abstract
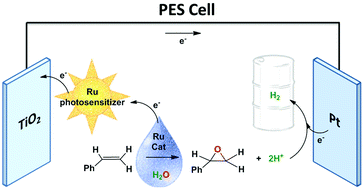
A dye-sensitized photoelectrosynthesis cell (DSPEC) has been prepared for the oxidation of alkenes to epoxides and evolution of hydrogen using water as an oxygen source and sunlight. A Ru oxidation catalyst, 2,2+, is used in the homogeneous phase in the anodic compartment to oxidize a water-soluble alkene, 4-styrene sulfonic acid (4-HSS), to the corresponding epoxide that in acidic solution is hydrolyzed to the diol (4-(1,2-dihydroxyethyl)-benzenesulfonic acid). Concomitantly, protons are generated that diffuse through a proton exchange membrane to a Pt cathode where they are transformed into hydrogen. Illumination under 1.5 AMG (100 mW cm−2) together with an external bias of 0.3 V vs. NHE after 24 h leads to the generation of 1.28 Coulombs together with the formation of 6.1 μmol of H2 at the cathodic compartment that corresponds to a faradaic efficiency of 92%. In addition 0.7 mM of the 4-HSS substrate has been oxidized at the anodic compartment with a conversion yield of 7%. The rate of hydrogen evolution is limited by the oxidation of the organic substrate, and the TOF for both reactions is measured to be 0.8 ks−1.

 Please wait while we load your content...
Please wait while we load your content...