Selective oxidative C–C bond cleavage of a lignin model compound in the presence of acetic acid with a vanadium catalyst†
Abstract
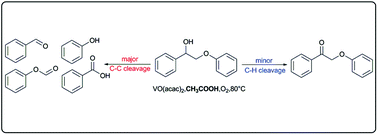
Selective transformation of lignin into value-added aromatics is highly attractive and rather challenging. Catalytic oxidative degradation provides a promising approach to obtain aromatics from lignin while preserving the benzene units. In this study, vanadium-catalyzed aerobic oxidation of 2-phenoxy-1-phenylethanol was studied as a lignin model compound. The solvent played a significant role in the distribution of oxidation products. In the presence of acetic acid, oxidative C–C bond cleavage was preferred, while oxidation products via C–H bond cleavage were rather limited. A one-electron transfer process was involved in the oxidation reaction. The occurrence of vanadium(V) was detected, and the carboxylic group could coordinate to vanadium(V) through exchange with the acetylacetonato ligand during oxidation in the presence of acetic acid.
- This article is part of the themed collection: Lignin chemistry and valorisation

 Please wait while we load your content...
Please wait while we load your content...