Near-to-eutectic mixtures as bifunctional catalysts in the low-temperature-ring-opening-polymerization of ε-caprolactone†
Abstract
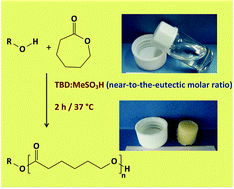
We have investigated the ring-opening polymerization (ROP) of ε-caprolactone using mixtures of methanesulfonic acid and the guanidine 1,5,7-triazabicyclo[4.4.0]dec-5-ene as the catalyst. Our interest in these mixtures is based on the capability of both acids and bases to behave as bifunctional catalysts; the former by the combined action of acidic hydrogens and basic oxygens, and the latter by the hydrogen-bond acceptor and donor features of, respectively, a basic nitrogen center and an ortho-hydrogen atom. We found that these compounds are capable of forming eutectic mixtures at a certain molar ratio. Upon the use of these mixtures – e.g. with molar ratios near to the eutectic one – as catalysts, neither further solvents nor initiators were required to carry out the ROP of ε-caprolactone. The resulting polycaprolactones (PCLs) were highly crystalline (more than 87%) and exhibited an excellent capability to support the growth of murine L929 fibroblasts. We consider that the preparation of biocompatible PCLs at physiological temperatures and in the absence of reagents other than the monomer and the catalyst offers an interesting alternative to both the self-cross-linked oligomers/macromers of acrylate-based PCL-derivatives and the pH/temperature-sensitive PCL copolymers used to date as injectable biomaterials.

 Please wait while we load your content...
Please wait while we load your content...