Extraction and separation of neodymium and dysprosium from used NdFeB magnets: an application of ionic liquids in solvent extraction towards the recycling of magnets
Abstract
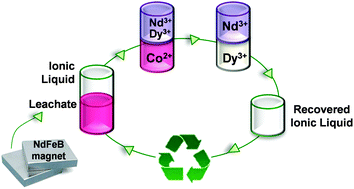
A procedure for the efficient extraction and separation of rare earths and other valuable elements from used NdFeB permanent magnets is presented. In a first step, an iron free leachate is prepared from an used magnet using nitric acid. Cobalt is separated through a liquid–liquid extraction in aqueous nitrate media using as organic phase the ionic liquid trihexyl(tetradecyl)phosphonium nitrate which is easily prepared from the commercially available ionic liquid trihexyl(tetradecyl)phosphonium chloride (Cyphos® IL 101). Afterwards neodymium and dysprosium are successfully separated using ethylenediaminetetraacetic acid (EDTA) as a selective complexing agent during liquid–liquid extraction with the same ionic liquid. Different parameters of the separation process such as shaking speed, time, temperature, pH effect and concentration of complexing agents were optimized. The designed process allowed the separation of these three elements efficiently in few steps. The separated rare earths and cobalt were precipitated with oxalic acid and then calcined in order to form the oxides. Nd2O3, Dy2O3 and CoO were obtained with purities of 99.6%, 99.8% and 99.8%, respectively. Recycling of the employed ionic liquid for reuse in rare earths separation was also demonstrated.

 Please wait while we load your content...
Please wait while we load your content...