An aza-Michael addition protocol to fluoroalkylated β-amino acid derivatives and enantiopure trifluoromethylated N-heterocycles†
Abstract
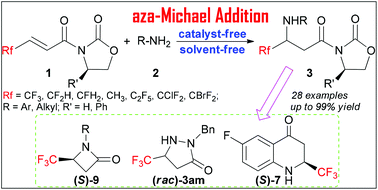
The aza-Michael reaction with β-fluoroalkylated acrylates provided the corresponding fluoroalkylated β-amino acid derivatives in up to 99% yield under catalyst- and solvent-free conditions. An enantioenriched β-trifluoromethylated β-amino acid was obtained in good yield through a scale-up diastereoselective aza-Michael addition, which facilitated the installation of enantiopure trifluoromethylated analogues of β-lactam and dihydroquinolin-4-one.

 Please wait while we load your content...
Please wait while we load your content...