Desulfurization of diesel fuel with nickel boride in situ generated in an ionic liquid†
Abstract
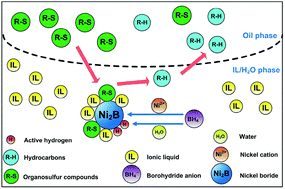
In order to improve the desulfurization efficiency, an ionic liquid (IL) was used as the solvent for the desulfurization of diesel fuel with nickel boride. The nickel boride prepared in IL–H2O showed high specific surface area. The desulfurization efficiency of model organosulfur compounds in this work was higher than that in the previous studies. The desulfurization reactivity of model organosulfur compounds followed the order of BT (DBT) > 3-MBT > 4,6-DMDBT. Furthermore, the products of model organosulfur compounds after desulfurization were analyzed by GC/MS and their corresponding reaction routes were proposed. The effectiveness of nickel salts followed the order of NiCl2 (Ni(OAc)2) > NiSO4 > Ni(NO3)2. The desulfurization efficiency of model diesel fuels reached 90.6% under the conditions of B/S molar ratio = 9, Ni(OAc)2/S molar ratio = 3, oil/IL volume ratio = 3, water content in IL = 5%, and reaction time = 50 min. ILs maintained their original structures after regeneration. Finally, the desulfurization of real diesel fuel was carried out and a desulfurization efficiency of 88.6% was obtained in 50 min.

 Please wait while we load your content...
Please wait while we load your content...