Direct access to 1,2,3-triazoles through organocatalytic 1,3-dipolar cycloaddition reaction of allyl ketones with azides†
Abstract
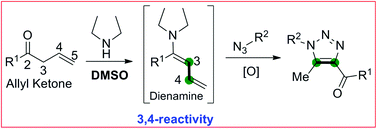
A general organocatalytic 1,3-dipolar cycloaddition reaction between allyl ketones and various azides is reported. The reaction is catalyzed by a secondary amine to generate substituted 1,2,3-triazoles with high levels of regioselectivity.

 Please wait while we load your content...
Please wait while we load your content...