The replacement for petrolatum: thixotropic ethylcellulose oleogels in triglyceride oils
Abstract
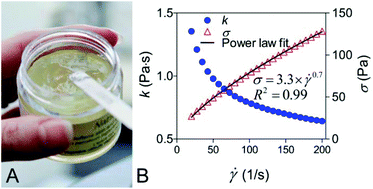
An ethylcellulose (EC) organogel was engineered with thixotropic properties in order to replace petrolatum, the petroleum jelly used extensively as a structuring agent in cosmetics, and the main component of Vaseline™. EC has been used to form oleogels with vegetable oils as the solvent phase. These gels tend to set at very high temperatures and, once set, can be irreversibly broken by shear forces such as those present during manufacture and use. To overcome this, thixotropic EC oleogels were produced that can recover all of their viscosity after removal of the shear. By changing the solubility parameters of the oil phase, particularly by matching the Hansen hydrogen bonding solubility parameter of the oil phase to that of EC, such as by addition of glycerol monooleate, the solubility of the EC in the oil can be increased. This increase in solubility leads to the production of fully thixotropic gels with viscosities that can be tailored by altering the EC concentration or viscosity. Furthermore, a gel made with 5–8% EC 10 cP and 40–50% glycerol monooleate in a variety of oils has favourable texture and water vapour barrier properties which make it a good candidate for use in cosmetics as a replacement for petroleum jelly.

 Please wait while we load your content...
Please wait while we load your content...