A procedure for the preparation of Ti-Beta zeolites for catalytic epoxidation with hydrogen peroxide†
Abstract
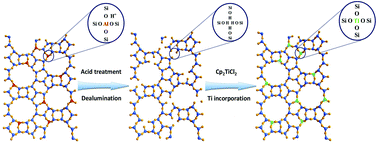
Ti-Beta zeolite has been successfully prepared via a reproducible and scalable two-step post-synthesis strategy, which consists of creating vacant T sites with associated silanol groups by dealumination of H-Beta and subsequent dry impregnation of the resulting Si-Beta with titanocene dichloride. The mechanism of Ti incorporation into the framework of Beta is investigated by diffuse reflectance infrared Fourier transform (DRIFT) and multinuclear solid-state nuclear magnetic resonance (SSNMR) spectroscopy. Characterization results obtained from diffuse reflectance ultraviolet-visible (UV-vis) and X-ray photoelectron spectroscopy (XPS) reveal that the majority of incorporated Ti species exist in the form of isolated tetrahedrally coordinated Ti(IV) in the zeolite framework while a minority exists in the form of isolated octahedrally coordinated Ti(VI) at framework or extra-framework positions. The obtained Ti-Beta zeolites are highly active and selective catalysts for the epoxidation of unsaturated ketones, e.g. 2-cyclohexen-1-one, with hydrogen peroxide as an oxidant. A quasilinear correlation between the epoxidation rate and the number of framework Ti(IV) species could be drawn evidencing that these Ti(IV) species are responsible for the epoxidation activity of the Ti-Beta zeolites under study. The impact of preparation parameters and reaction conditions on the catalytic performances of the Ti-Beta zeolites in the epoxidation of unsaturated organic compounds with hydrogen peroxide is discussed in detail.

 Please wait while we load your content...
Please wait while we load your content...