Highly efficient and selective photocatalytic hydrogenation of functionalized nitrobenzenes†
Abstract
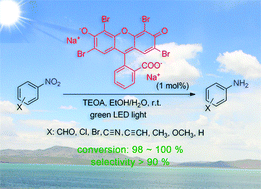
We report a simple but efficient photocatalytic nitrobenzene reduction method employing eosin Y as the photocatalyst and TEOA as the reducing agent. With green LED light irradiation, the nitro group in the nitrobenzenes containing other reducible groups was chemoselectively reduced into an amino group, and the corresponding anilines were isolated in quantitative yields. The photoinduced electron transfer mechanism suggests that the high chemoselectivity originates from the better electron-withdrawing ability of the nitro group.

 Please wait while we load your content...
Please wait while we load your content...