Depolymerisation of condensed tannins in ethanol as a gateway to biosourced phenolic synthons
Abstract
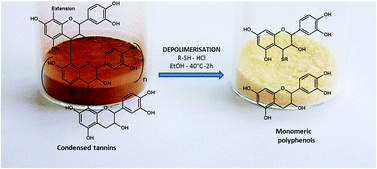
Plant polyphenols are potential sources of substitutes for phenolic petrochemicals, such as bisphenol A. Among them, condensed tannins are the most abundant after lignins in terrestrial plants. They are especially encountered in agro-industrial residues (i.e. fruit marc) and unprocessed biomass (i.e. barks, needles, and leaves). However, this class of biopolymers requires a depolymerisation step prior to their industrial use as fine chemicals. In this work, phenolic synthons were produced by depolymerisation of an industrial grape seed extract of condensed tannins at the multigram scale. The optimization of the depolymerisation reaction through the selection of the most suitable solvent, nucleophilic agent and temperature allowed the reaction to be carried out at mild temperature (40 °C), in two hours, using ethanol as a solvent. The isolation of compounds of interest was performed by precipitation in ethyl acetate followed by a simple adsorption/desorption step on a polyamide cartridge using ethanol as an eluent.

 Please wait while we load your content...
Please wait while we load your content...