Merging the ring opening of benzoxazoles with secondary amines and an iron-catalyzed oxidative cyclization towards the environmentally friendly synthesis of 2-aminobenzoxazoles†
Abstract
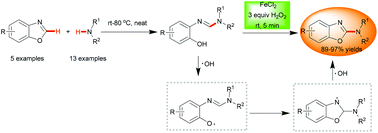
A facile and environmentally friendly method was developed through merging the ring opening of

 Please wait while we load your content...
Please wait while we load your content...