l-Proline supported on ionic liquid-modified magnetic nanoparticles as a highly efficient and reusable organocatalyst for direct asymmetric aldol reaction in water†
Abstract
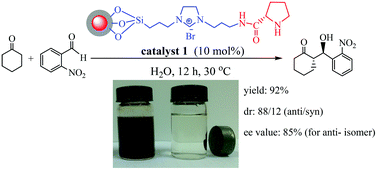
Grafting L-proline on imidazolium-based ionic liquid (IL)-functionalized magnetic nanoparticles afforded a magnetically recoverable L-proline catalyst. Characterization technologies suggested the presence of an L-proline backbone, an IL linker, and a magnetic ferrite core in the catalyst. The resulting L-proline catalyst was efficient for direct asymmetric aldol reaction in water without the need for organic solvents and co-catalysts. Such efficiency is attributed to the fact that the IL moiety facilitated the accessibility of hydrophobic reactants to active sites in water and stabilized the formed enamine intermediate during the reaction. High activity (yield = 92%), diastereoselectivity (dr; 88/12) and enantioselectivity (ee; 85%) were obtained using 10 mol% of a catalyst for the reaction between cyclohexanone and 2-nitrobenzaldehyde within 12 h, where the pristine L-proline and IL-free counterpart were almost inactive. The catalyst was easily separated using a permanent magnet externally and can be reused several times without significant loss of activity.

 Please wait while we load your content...
Please wait while we load your content...