Substrate imprinted lipase nanogel for one-step synthesis of chloramphenicol palmitate†
Abstract
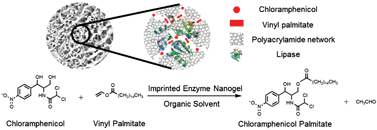
Enzymatic catalysis with high enantio- and regio-selectivity, which is attractive for green synthesis of chemicals, often suffers from low activity in organic solvents utilized as reaction media. Here, we describe a ‘substrate-imprinted’ lipase nanogel that displays high activity in organic solvents. The first step was to encapsulate lipase into polyacrylamide nanogel by an aqueous in situ polymerization. Then the lipase nanogel was lyophilized in the presence of palmitic acid, a substrate of lipase, followed by extraction with petroleum ether to remove palmitic acid from the lyophilized lipase nanogel. The imprinting treatment increased the adsorption capacity of palmitic acid by 2.9-fold and the apparent activity by 2-fold in catalyzing the transesterification reaction between para-nitrophenyl palmitate and ethanol. The effects of solvent and temperature on the yield and selectivity of the enzymatic synthesis of chloramphenicol palmitate were examined, respectively. One-step synthesis of chloramphenicol palmitate with the imprinted lipase nanogel gave a yield of ∼99% and a purity of ∼99% within 12 hours at 20 °C, whereas the imprinted free lipase gave a yield below 60% in 20 hours. The high activity and selectivity make the substrate-imprinted enzyme nanogel an attractive catalyst for green synthesis of chemicals having complex structures.

 Please wait while we load your content...
Please wait while we load your content...