Laccase-catalyzed oxidative phenolic coupling of vanillidene derivatives†
Abstract
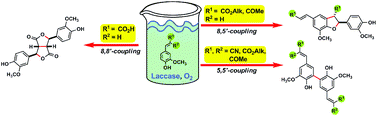
The laccase-catalyzed oxidative phenolic coupling of vanillidene derivatives using aerial oxygen as the oxidant has been developed. Depending on the substitution pattern of the vanillidene double bond of the substrate, either dilactones, dihydrobenzo[b]furans or

 Please wait while we load your content...
Please wait while we load your content...