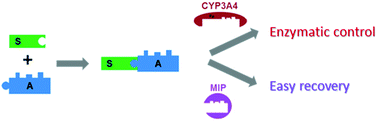
Auxiliary-directed biocatalysis is a powerful and relatively benign tool for chemical synthesis. We recently reported the use of theobromine as a chemical auxiliary for directing CYP3A4-catalyzed oxidations with predictable regio-, chemo- and stereo-selectivity (J. Am. Chem. Soc., 2011, 133, 7853). This highly successful strategy enabled the direct, regio- and stereo-selective hydroxylation of unactivated C–H bonds. However, recovering the products and starting materials from the resulting biocatalytic mixtures has proven challenging. To address this issue, we capitalized on the chemical auxiliary present on the starting material and enzymatic reaction products. The chemical auxiliary was used as a template to generate molecularly imprinted polymers (MIPs). When used in solid-phase extractions, the MIPs reported here allow for the recovery (>90%) of theobromine-containing products and starting materials from complex mixtures such as cellular membrane extracts. This strategy, which avoids the use of large amounts of organic solvents, represents an effective, easily-tailored, and reusable way to improve the recovered yield of biocatalytic transformations while extending the usefulness of a chemical auxiliary.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...