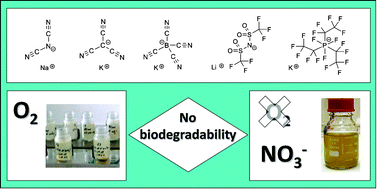
The present study deals with the primary biodegradability of ionic liquids in order to obtain a greater insight into their fate under different environmental conditions. The focus was thereby on the biodegradation potential of ionic liquid anions when undergoing aerobic and anaerobic biological waste water treatment. Five technologically relevant fluoroorganic and cyano-based ionic liquid anions were investigated as alkaline salts (Li (CF3SO2)2N, K (C2F5)3PF3 and Na N(CN)2, K C(CN)3, K B(CN)4 respectively). Their biodegradability was determined in activated sludge over a period of around 60 days by specific analysis of the anion using ion chromatography. Additionally, the antimicrobial activity of the test compounds towards the activated sludge organisms was tested in inhibition studies. Because of the technologically desirable chemical, thermal and electrochemical stability of these anions, their biodegradability is questioned. The results seem to support the hypothesis: although the concentrations used did not inhibit the inoculum, none of these anions could be biodegraded under either aerobic or denitrifying conditions. The present paper provides information concerning the biodegradability of ionic liquids in waste water treatment plants and gives a first systematic view of the aerobic and anaerobic biodegradability of fluoroorganic and cyano-based ionic liquid anions and therefore supports further hazard assessment.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...