l-Proline-catalysed sequential four-component “on water” protocol for the synthesis of structurally complex heterocyclic ortho-quinones†
Abstract
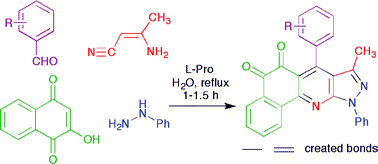
The L-proline-catalyzed synthesis of 7-(aryl)-8-methyl-10-phenyl-5H-benzo[h]pyrazolo-[3,4-b]quinoline-5,6(10H)-diones via the four-component sequential reaction of ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bonds in a single operation. The environmental advantages of the method include short reaction time, excellent yield, easy work-up, and the absence of
N bonds in a single operation. The environmental advantages of the method include short reaction time, excellent yield, easy work-up, and the absence of

 Please wait while we load your content...
Please wait while we load your content...