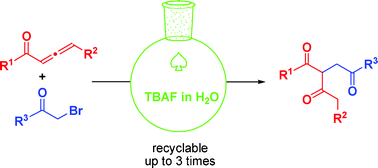
Tandem reaction of 1,2-allenic ketone with α-halo ketone or α-halo ester in water: an efficient and sustainable synthesis of 1,3,4′-tricarbonyl compounds†
Abstract
An efficient and sustainable synthesis of the otherwise difficult to obtain 1,3,4′-tricarbonyl compounds through a water mediated, TBAF·3H2O promoted unprecedented tandem reaction of 1,2-allenic ketone with α-halo

 Please wait while we load your content...
Please wait while we load your content...