From NaHCO3 into formate and from isopropanol into acetone: Hydrogen-transfer reduction of NaHCO3 with isopropanol in high-temperature water†
Abstract
In high-temperature
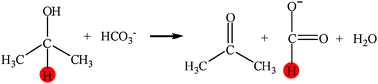
- This article is part of the themed collection: 2nd Asia Pacific Conference on Ionic Liquids and Green Processes

 Please wait while we load your content...
Please wait while we load your content...