Efficient and ‘green’ microwave-assisted synthesis of haloalkylphosphonates via the Michaelis–Arbuzov reaction†
Abstract
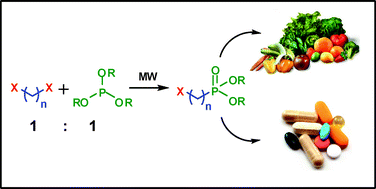
This paper deals with a novel, efficient and environmentally friendly synthesis of dialkyl haloalkylphosphonates via a microwave-assisted Michaelis–Arbuzov reaction. The approach is solventless, requires only one equivalent of each of the starting compounds, and provides high yields of pure products from which the impurities are easy to remove. The process has been optimised for batch and flow reactors and is especially profitable for the production of key intermediates in synthesis of

 Please wait while we load your content...
Please wait while we load your content...