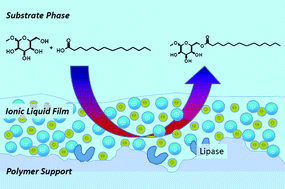
A homologous series of biosurfactants has been synthesized by a novel sustainable biotransformation technique and compared with three other enzymatic processes. 6-O-Alkanoyl-methyl-α-D-glucopyranosides were obtained by lipase mediated esterification of methyl-α-D-glucopyranoside with capric acid C10:0, lauric acid C12:0, myristic acid C14:0, palmitic acid C16:0, and oleic acid C18:1. Solvent free transformations were compared with the use of ionic liquids and organic solvents. The lipase from Candida antarctica B, immobilized on macroporous acrylic acid beads (Novozyme 435), was employed either untreated or coated with small amounts of ionic liquids. This resulted in superior efficiencies (80%) with 1-butyl-4-methylpyridine hexafluorophosphate [4bmpy][PF6] and broader substrate tolerance in comparison to solvent free transformation. The results show a positive correlation with increasing polarity of the ionic liquids used as liquid film-coating, which was in opposition to the use of the same ionic liquid as solvent. The analysis of the ionic liquid film coated catalyst carriers was performed by optical and scanning electron microscopy (SEM).
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...