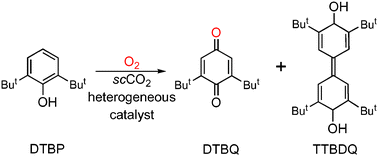
Development of processes that utilize heterogeneous catalysis in environmentally beneficial media is of fundamental and practical importance. The oxidation of 2,6-di-tert-butylphenol (DTBP) to 2,6-di-tert-butyl-1,4-benzoquinone (DTBQ) and 3,5,3′,5′-tetra-tert-butyl-4,4′-diphenoquinone (TTBDQ) has been investigated to evaluate the factors necessary to achieve high product conversion and selectivity in various media. A series of porous materials with immobilized Co(II) complexes served as catalysts and their reactivities using O2 as the terminal oxidant were screened in neat acetonitrile, supercritical carbon dioxide (scCO2), and CO2-expanded acetonitrile. The highest conversions were found with the catalysts that had high affinity for dioxygen. Moreover, the greatest conversions (∼60%) were obtained when reactions were done in scCO2, which is attributed to improved mass transfer of O2 and substrates through the porous catalysts. Furthermore, the heterogeneous catalysts can be recycled with some loss of activity (∼30%) after three cycles; nonetheless these results suggest that the polymer hosts efficiently protect the immobilized catalytic sites from destructive bimolecular routes.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...