Direct mono-N-alkylation of amines in ionic liquids: chemoselectivity and reactivity†
Abstract
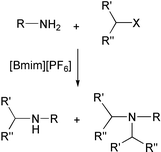
A simple method for the N-alkylation of primary amines was developed using ionic liquids as solvent in order to prepare secondary amines selectively. In ionic liquids overalkylation of the initially produced secondary amines is in general markedly reduced. Various amines, alkyl halides and sulfonates were examined. The observed selectivities between mono- and dialkylation are typically on the order of 9∶1, or higher. Only in the cases of allyl or benzyl bromides does the reaction give the corresponding tertiary amines exclusively. The relative nucleofugality of chloride, bromide, iodide and tosylate with several primary amines was also evaluated, as well as the effect of caesium hydroxide.

 Please wait while we load your content...
Please wait while we load your content...