Exhaustive hydrodechlorination of chlorinated aromatic environmental pollutants to alicyclic compounds
Abstract
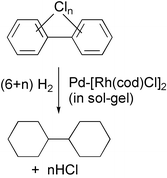
A combined Pd–Rh catalyst obtained by co-entrapment of metallic palladium and soluble [Rh(cod)Cl]2 within a silica sol–gel matrix exhaustively detoxifies chlorinated aromatic pollutants not only by total removal of the chlorine, but also by the total reduction of the aromatic moieties to alicyclic rings. The list of chloroarenes which have been successfully destroyed by the catalysts includes most of the major pollutants which are of public concern, namely chlorinated dibenzo[b,e]dioxin and dibenzofuran, PCBs, DDT, Hexachlorophene and 2,4,5-T. The reactions take place under the relatively mild conditions of 80–100 °C under 27 atm H2. The immobilised catalyst is leach proof in a variety of solvents and can be reused in several runs. The high activity of the combined Pd–Rh system is attributed to synergism between the two different metal atoms.

 Please wait while we load your content...
Please wait while we load your content...