Zero-valent metal accelerators for the dechlorination of pentachlorophenol (PCP) in subcritical water
Abstract
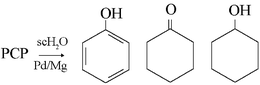
Preliminary trials with 0.5 mg pentachlorophenol (PCP) in the presence/absence of 50 mg of 325-mesh Ag0/Fe0 (2% w/w) granules, demonstrated that the extent of dechlorination increased with both increased reaction time (0.5–2 h) and increased temperature (200–350 °C) but that recoveries of products were incomplete. Whereas reaction (200 °C) in the absence of metal accelerator furnished only unreacted PCP after 2 h and a mixture of tetrachloro species and substrate (∼50%) after 4 h of reaction, timed trials in the presence of Fe0 or 2% (w/w) Ag0/Fe0 bimetallic mixture resulted in more extensive dechlorinations. Only sequential dechlorinations were observed for iron-based accelerators and the dechlorination route was consistent with relief of steric strain as the dominant influence that determined the decomposition products. There was no appreciable difference in the course of the dechlorinations between the Fe0 and the 2% (w/w) Ag0/Fe0 bimetallic mixture but differences in the rates of reaction were evident. Dechlorinations with magnesium-based bimetallic accelerators were more extensive, more rapid and both concerted and stepwise reactions were observed. With 100 mg Pd0/Mg0, 63% of products consisted of phenol plus cyclohexanone and a further 9% was o-chlorophenol yet 13% were tetrachloro species. By contrast, an equal quantity of Mg0 alone mediated only partial dechlorination. The dechlorinations represent a detoxification—the toxiforic chloro substituents of the substrate are reduced to innocuous chloride while the sacrificial Mg metal is oxidised to Mg2+.

 Please wait while we load your content...
Please wait while we load your content...