Examining the inhibitory potency of food additive fast green FCF against amyloid fibrillogenesis under acidic conditions†
Abstract
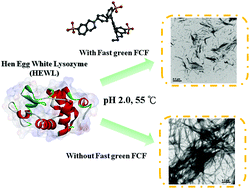
More than thirty human proteins and/or peptides can fold incorrectly to form amyloid deposits associated with several protein aggregation diseases. No cure is currently available for treating these diseases. This work is aimed at examining the inhibitory potency of fast green FCF, a biocompatible dye, toward the fibrillogenesis/aggregation of lysozyme. As verified by ThT binding assay along with transmission electron microscopy, fast green FCF was observed to suppress the generation of lysozyme fibrils in a concentration-dependent manner. We next used circular dichroism absorption spectroscopy, ANS fluorescence spectroscopy, and SDS-PAGE to characterize the structural alterations in lysozyme samples upon the addition of fast green FCF. Furthermore, experiments with the addition of fast green FCF at different time points of incubation showed that fast green FCF also exhibited disaggregating activity against the preformed/existing lysozyme fibrils. We believe that the results from this study suggest a potential therapeutic role of biocompatible molecules in treating or preventing protein aggregation diseases.

 Please wait while we load your content...
Please wait while we load your content...