Emerging investigators series: ultraviolet and free chlorine aqueous-phase advanced oxidation process: kinetic simulations and experimental validation†
Abstract
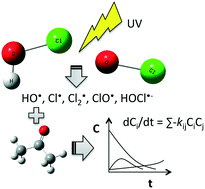
An emerging advanced oxidation process uses ultraviolet light and free chlorine to produce active hydroxyl radicals and chlorine-derived radicals to degrade a variety of organic compounds in water. The use of free chlorine and reactivity of chlorine-derived radicals with many organic compounds have raised concerns about the potential formation of toxic degradation byproducts, e.g., chlorinated byproducts. An elementary reaction-based kinetic model is an attractive and promising approach to predict the degradation of a target organic compound and its degradation products and to provide mechanistic insight into the reaction mechanisms. We developed a UV/free chlorine elementary reaction-based kinetic model for a test compound, acetone, and its transformation products. The elementary reaction pathways were predicted by quantum mechanical calculations, and the reaction rate constants were predicted using previously developed linear free energy relationships. Ordinary differential equations were generated and numerically solved to obtain the time-dependent concentration profiles of acetone and its transformation products. Our experimental results were used to validate the model.
- This article is part of the themed collections: Emerging Investigator Series and Ultraviolet-based Advanced Oxidation Processes (UV AOPs)


 Please wait while we load your content...
Please wait while we load your content...
