Coagulation of colloidal particles with ferrate(vi)†
Abstract
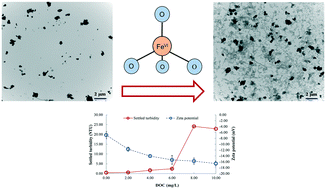
Coagulation and chemical oxidation have long been recognized as two major mechanisms of ferrate(VI) (i.e. FeO42−, an oxyanion containing Fe(VI)) in its environmental applications. Although ferrate(VI) oxidation of various contaminants has been extensively studied, few efforts were made to appreciate the mechanisms and behaviors of ferrate(VI)-driven coagulation in water. In this study, coagulation of colloidal kaolin particles with ferrate(VI) in simulated natural water was investigated under the conditions related to drinking water treatment (initial turbidity 25.00 NTU, 0.0–9.0 mg L−1 Fe(VI), pH 7.5, and 0.50–10.00 mg L−1 DOC). Fe(III) produced from Fe(VI) reduction initiated in situ coagulation. Lower minimum effective iron doses (MEIDs) were observed for Fe(VI) coagulation than for direct Fe(III) coagulation at pH 6.5 and 7.5 (DOC = 2.00 mg L−1), at which the colloids were captured by iron precipitates principally via sweep coagulation. A citrate–ascorbate iron extraction method was used to reveal that Fe(VI) resultant flocs were composed of both amorphous and crystalline iron with an amorphous to crystalline Fe ratio of approximately 2.3 : 1.0 (pH 7.5, 3.0 mg L−1 Fe(VI), and 2.00 mg L−1 DOC). Ferrate(VI) oxidation transformed natural organic matter (NOM) preferentially into more hydrophilic compounds, which have lower affinity with colloids and thus are less adsorbed on colloids to produce a less negatively charged surface. Therefore, ferrate(VI) oxidation potentially promoted the aggregation of colloids through the alleviation of electrostatic repulsion. However, NOM at a high concentration (8.00–10.00 mg L−1 DOC in this study) could prevent the agglomeration of small iron oxide particles through the formation of a negatively charged NOM coating via adsorption, thereby preventing the growth of flocs. Ferrate(VI) coagulation, when combined with ferrate(VI) oxidation, provides a more viable treatment option to address multiple water pollution in raw water, e.g. the presence of colloidal algal cells and dissolved algal toxins in water during a harmful algal bloom. The dual treatment mechanisms enable a very effective treatment design with an economical physical footprint for supporting a sustainable municipal drinking water supply.


 Please wait while we load your content...
Please wait while we load your content...