Design strategies for SCR catalysts with improved N2 selectivity: the significance of nano-confining effects by titanate nanotubes
Abstract
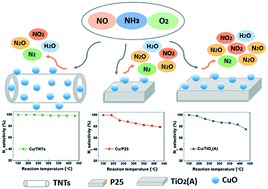
Copper has been investigated as a promising alternative for SCR reactions. However, most Cu-based catalysts supported on zeolites, alumina or titania produced a certain amount of undesired N2O by-product. Herein, we report a strategy to tune the redox properties of copper oxides via confinement within titanate nanotubes (Cu/TNTs) and investigate their SCR activity as well as the N2 selectivity. The Cu/TNT catalyst showed an excellent NO reduction performance (above 90%) in the temperature window of 300–450 °C and the N2 selectivity could exceed 98% among the whole reaction temperature range of 150–470 °C, with a negligibly low concentration of N2O being detected. After systematic characterizations, the tuning of the chemical state of copper and oxygen, the remarkable adsorption capability, the accelerated oxidation of Cu+ to Cu2+, the lower level of NH3 oxidation and ultimately the tuning of redox properties were discovered. This work could provide a new approach to design SCR catalysts with superior catalytic reduction performance as well as excellent N2 selectivity.


 Please wait while we load your content...
Please wait while we load your content...