Nanoceria biodistribution and retention in the rat after its intravenous administration are not greatly influenced by dosing schedule, dose, or particle shape
Abstract
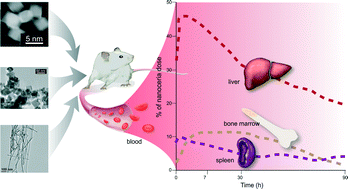
A 30 nm ceria was previously shown to be primarily cleared from systemic circulation into mononuclear phagocyte system organs after its intravenous (IV) administration, where it persisted for 90 days. The aims of these studies were to determine if the biodistribution, persistence, and toxicity of nanocerias are affected by dosing schedule, dose, or particle shape. Given the many demonstrated and ongoing uses of nanocerias; the multitude of applications under investigation; and the many sizes, shapes, and surface functionalizations of ceria; a better understanding of its fate is necessary to advance its safe use. Five and 30 nm cubic/polyhedral ceria and a ceria nanorod (9.9 × 264 nm average diameter and length) were IV infused into rats once. The 5 nm ceria dose was also infused daily for 5 consecutive days. The rats were terminated 1 h to 90 days later. Cerium was determined in multiple organs and blood. Compared to vehicle-infused controls, elevated cerium was seen in all sites. Liver, spleen, and bone marrow (mononuclear phagocyte system components), contained the largest percentage of the dose. When normalized to dose, and compared to results of prior work with these nanocerias, the distribution and retention of repeated and lower doses of 5 and 30 nm ceria and ceria nanorods were not greatly different from much higher doses of the 5 and 30 nm ceria. Higher doses resulted in a greater percentage of uptake by the spleen and bone marrow and a greater percentage of the ceria nanorod dose in the bone marrow 30 days after its administration than the other nanocerias. Overall these results suggest the biodistribution and retention of cerium after IV administration of different sizes, doses, dosing schedules, and nanoceria shapes are more similar than different.
- This article is part of the themed collection: Nanoceria Research

 Please wait while we load your content...
Please wait while we load your content...