Galena weathering under simulated acid rain conditions: electrochemical processes and environmental assessments
Abstract
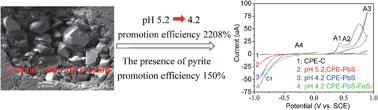
Galena can be easily weathered under acid rain conditions and causes environmental issues. This process, in nature, is an electrochemical process. In situ electrochemical technology and surface analysis technology were combined to investigate this weathering process. The experimental results showed that galena weathering under simulated acid rain (SAR) could cause an initial increase in Pb2+ ions and result in the formation of a passive S0 film, and the terminal product was SO42−. Increased acidity stimulates galena weathering, and the promotion efficiency was 2208% as the pH of the SAR decreased from 5.2 to 4.2. The presence of pyrite is favourable for galena weathering, and the promotion efficiency was 150% when contained 25% (wt%) pyrite. Galena will release 1.7 g per m2 per year Pb2+ to solution when the pH of acid rain is 5.2, and the released Pb2+ will reach 95.7 g per m2 per year when the pH of acid rain is 4.2 plus the galvanic effect of pyrite.


 Please wait while we load your content...
Please wait while we load your content...